Featured image: Crystals of the mineral barite from the deep ocean (Adapted from Kastner (1999)). These crystals precipitated in ocean sediments and are about 9 million years old, similar in age to some of the barite samples from the study discussed here.
Paper: A 35-million-year record of seawater stable Sr isotopes reveals a fluctuating global carbon cycle
Authors: Adina Paytan, Elizabeth M. Griffith, Anton Eisenhauer, Mathis P. Hain, Klaus Wallmann, Andrew Ridgwell
What do ancient ocean sediments and the walls around x-ray machines have in common? One possible answer? Sometimes the mineral barite is an important part of both! Barite (or barium sulfate) is able to block gamma and x-ray emissions, and therefore is sometimes used in high-density concrete in hospitals and laboratories. In the deep ocean, tiny crystals of barite naturally accumulate on the seafloor over time, particularly in regions ideal for this mineral formation where many decaying remains of organisms sink to the seafloor. The chemistry of this barite can give scientists clues into Earth’s past, which is what Adina Paytan and her colleagues did in this study.
One of the burning questions in paleoclimate science is how and why different components of Earth’s carbon cycle, such as atmospheric carbon dioxide, have changed over time. However, it is notoriously difficult to understand the whole carbon cycle, because there are lots of interacting processes in the cycle that are hard to measure today, let alone millions of years ago!
Paytan et al. tackled this confusion by recognizing that barite in ocean sediments is a key archive for isotopes of the element strontium, which gives information on multiple processes related to the past carbon cycle. She and her colleagues made two different measurements of strontium isotopes on the exact same barite crystals, one that quantifies the abundance of the radiogenic isotope (87/86 strontium isotopic ratio), and the other that quantifies the abundance of stable strontium isotopes (88/86 strontium isotopic ratio). They made these paired radiogenic and stable strontium isotope measurements from ocean sediments across multiple locations in the Pacific Ocean that span back to 35 million years ago.
Their measurement strategy was important in two main ways. First, their sediment samples were spread out geographically, and so tight correspondences between measurements from distant locations indicated that their conclusions indeed reflected changes in global climate processes. Second, each type of strontium isotope measurement illustrates different processes that affected the carbon cycle back in time. Measurements of radiogenic strontium isotope ratios (87/86) contain information on how much rock weathering occurred on the continents. This weathering is affected by temperature, precipitation, and erosion on land and impacts carbon content in the atmosphere and ocean. On the other hand, the authors show that stable strontium isotope ratios (88/86) primarily contain information on the overall strontium content of the ocean. Importantly, this strontium content is mainly affected by how many shallow-water (neritic) calcium carbonate minerals were deposited in the ocean, which represents a major geological reservoir of carbon. Their measurement of both radiogenic and stable strontium isotopes therefore meant that they could assess multiple avenues of changes in the carbon cycle over time.
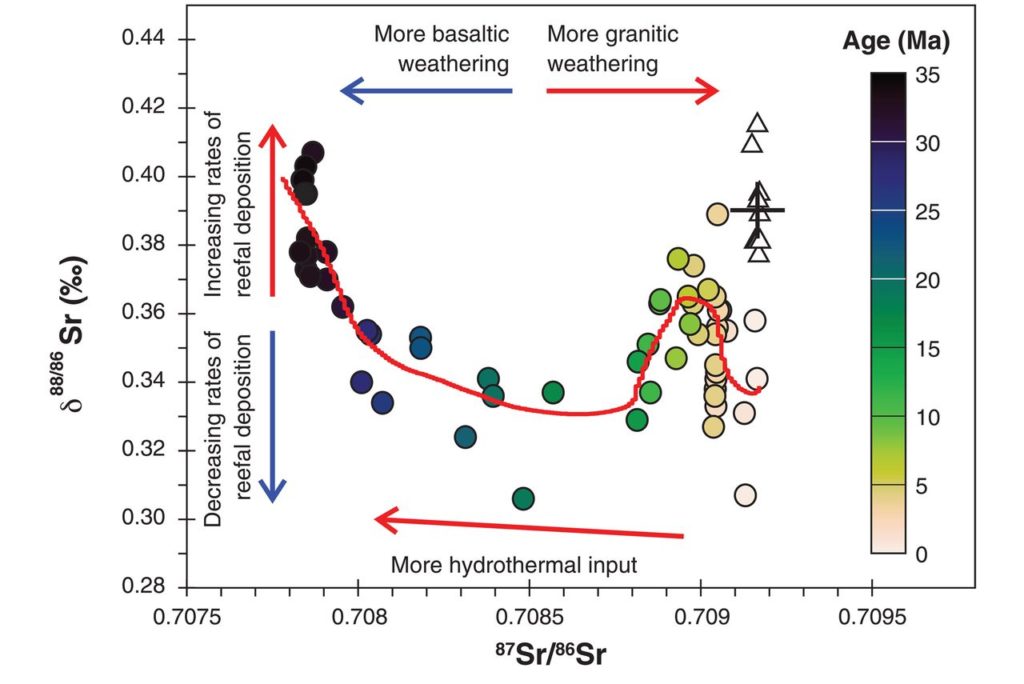
The authors’ data indicate considerable variability in the long-term carbon cycle (see figure above). For example, in the period 35 to 25 million years ago, they measured significant decreases in the stable strontium isotope values at the same time as a small change in radiogenic strontium isotopes. They suggest this change was due to a major decline in shallow-water carbonate deposition, possibly due to drops in sea level over this time. Then, from about 25 to 15 million years ago, stable strontium isotopes didn’t change much but radiogenic strontium isotopes increased substantially, which they say could indicate increased erosion of the Himalaya mountain range during this time (with not much change in carbonate deposition in the ocean). Further fluctuations in these strontium isotope trends from 15 million years ago to the modern day also similarly suggest changes in weathering on land, global sea level, and marine carbonate deposition.
The most unique part of this study is that Paytan and her coauthors applied a new two-pronged approach using both types of strontium isotope measurements to better constrain this important and often confusing area of paleoclimate research. The weathering of mountains in India and the deposition of calcium carbonate reefs near the equator might seem worlds apart, but barite from the deep ocean links them together and promises to teach us more about the ancient carbon cycle.
Buried treasure in the oceans: chemistry of small deep-sea crystals hints at past carbon cycling by Lloyd Anderson is licensed under a Creative Commons Attribution 4.0 International License.