Featured image: Example of the rock type Pegmatite. Here, crystals of the mineral tourmaline (light-dark green color), and crystals of the mineral lepidolite (pink-purple color) can be seen, sourced from Wikipedia. This file is licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
Paper: Episodes of fast crystal growth in pegmatites
Authors: Patrick R. Phelps, Cin-Ty A. Lee, Douglas M. Morton
Anyone who has ever wandered along a pebble-ridden beach or a mountainous trail has likely picked up a rock or two, and maybe these rocks contained an array of different crystals (see image above). Perhaps these rocks then skipped along the surface of a still lake, or made their way into the pockets of a snack-ridden backpack, either to never be seen again or to be added to an ever-growing rock collection. Yet, these little pieces of Earth’s history have the potential to do so much more. With the right tools, the crystals within these rocks can be used to inform us of the geological processes that have shaped our planet Earth.
In a recent study, Patrick Phelps and co-authors used Pegmatite, a type of crystalline rock, to investigate and challenge our understanding of how fast crystals can grow. Pegmatite is an example of a coarse-grained rock and is defined by having crystal lengths greater than 2.5cm (e.g., the diameter of a US quarter coin), and often much larger. The word Pegmatite itself is derived from Homeric Greek, πήγνυμι (pegnymi), and translates as “to bind together”, a reference to their interlocked nature (see Figure 1 below).

The formation of Pegmatites is generally associated with the cooling of molten rock (magma) and the formation of solids (crystals). Traditional thought and reasoning often attributes small crystal sizes to magma cooling over minutes to days (e.g., 0.5 oC to 15oC/min). Under these conditions the crystals simply do not have time to grow before the molten magma becomes a completely crystalline solid. The resulting crystals are therefore locked in the rock record at sizes from nanometers to millimeters. Conversely, larger crystal sizes (maximum lengths of several centimeters in size and larger) are associated with slower cooling rates on the order of tens of thousands to hundreds of thousands of years. As a result of different cooling environments, the temperatures of which may vary due to depth within the Earth (cooler at shallower depths), a wide range of fine-grained to coarse-grained rocks exist. Despite this paradigm, some of our planets largest crystals are known to have grown over relatively short cooling histories, posing a “Pegamatite Puzzle”.

To address the so-called puzzle, the recent work of Phelps et al. (2020) examined the chemical composition of quartz, one of the crystals in Pegmatites, to better understand the conditions that naturally promote the rapid growth of large crystals over short timescales. Phelps et al. (2020) specifically focused on crystals of the mineral quartz from the Stewart Pegmatite of southern California. First, quartz crystals were imaged revealing three distinct growth zones (see Figure 3 below). Then, this high-resolution imaging was used to guide subsequent analyses of the quartz crystals for their chemical compositions at the micrometer scale (see Figure 3 below).
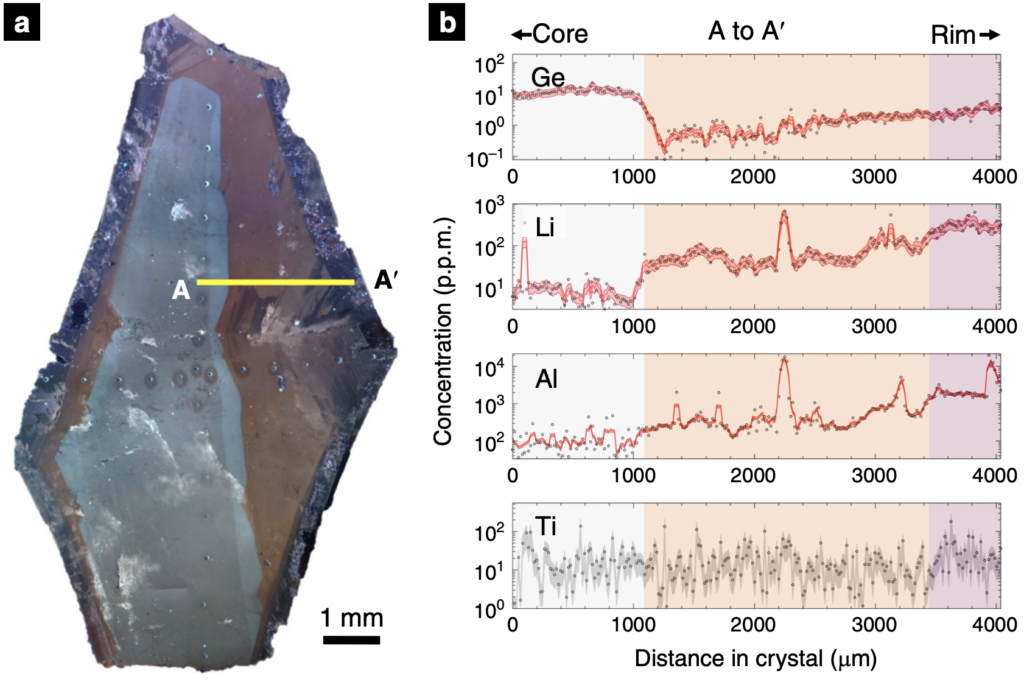
As shown above in Figure 3, the elements across the quartz crystals are broadly related to the imaged growth zones. The distribution of elements in the quartz crystal is ultimately determined by three factors, as discussed by lead author Phelps: 1) how easily the element is incorporated into the quartz crystal structure, 2) the rate of crystal growth, and 3) how quickly or slowly the element itself reaches the quartz crystal in system (the diffusivity). By integrating the observed element concentrations with knowledge of each element’s diffusion within the quartz crystal, Phelps et al. (2020) developed a mathematical model which translated the distribution of elements into a crystal growth rate. From this, the Stewart Pegmatite quartz crystals of southern California were revealed to have grown in stages. The initial growth was at the rate of millimeters per day, and later growth at the crystal edge was meters per day. These rapid rates are in stark contrast to the timescales of thousands of years that have traditionally been attributed to the formation of pegmatites and other coarse-grained crystalline rocks.
The work presented in Phelps et al. (2020) not only provides a new tool with which to decipher the crystal record of coarse-grained (and fine-grained) rocks it also provides new constraints on the rates of geological processes which operate on our planet. Therefore, all readers are encouraged to pick up a rock when they find themselves wandering along a shoreline. Take a moment to identify any crystals that are present and consider how long it took them to grow. They may have grown faster than you think.

Cooking up crystals in record time by Claire McLeod is licensed under CC BY-SA 4.0

