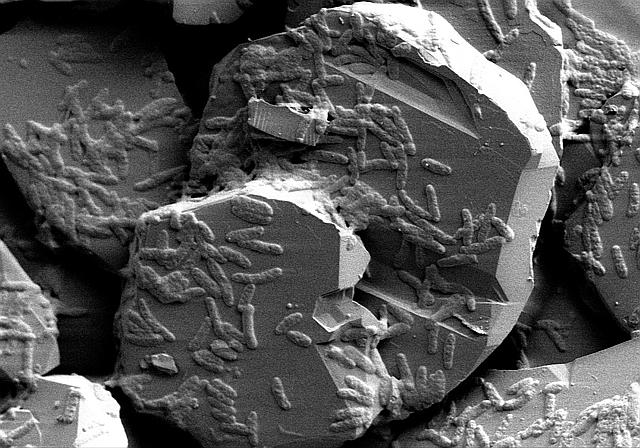
Featured Image: Shewanella putrefaciens CN-32 (a microbe capable of eating iron) on hematite (a rock containing iron). Image courtesy Environmental Molecular Sciences Laboratory (EMSL). Used with permission.
Paper: Organic matter mineralization in modern and ancient ferruginous sediments
Authors: André Friese, Kohen Bauer, Clemens Glombitza, Luis Ordoñez, Daniel Ariztegui Verena B. Heuer, Aurèle Vuillemin, Cynthia Henny, Sulung Nomosatryo, Rachel Simister Dirk Wagner, Satria Bijaksana, Hendrik Vogel, Martin Melles, James M. Russell, Sean A. Crowe, Jens Kallmeyer
Just as a crow may use a rock to crack a nut, certain microbes can use solid iron to crack open methane. This consumption limits the amount of methane lost from lakes into the atmosphere, making it a crucial process in mitigating production of greenhouse gasses. These microbes are abundant in freshwater sediments, and their specialized mechanism for cracking open methane is most likely one of the oldest metabolisms on Earth, providing a modern-day window into the past.
But in an astonishing discovery, researchers have identified a lake in Indonesia, Lake Matano, where these specialized microbes are unable to use iron to access organic matter or methane. Since Lake Matano seemingly has the perfect conditions to limit the escape of methane, why are these microbes not feasting? Friese and colleagues developed a rigorous extraction that gains greater insight into the iron present in these sediments and why it can’t be used by methane-cracking microbes.
The answer comes from physical inaccessibility. The iron that microbes use comes from solid rocks found within the lake sediments. Typically, the microbe is able to extend a long arm (known as a nanowire) to touch the rock and transfer electricity from the cell to the rock. This gives the microbe the energy to then break open the strong chemical bonds found in organic matter or methane. When the microbe breaks open the methane, they are rewarded with energy they can use in their cells. Friese and coworkers sampled the iron present in the sediments of Lake Matano and found that the iron was mostly locked up in rocks that the microbes cannot access easily with a nanowire. These rock formations make it difficult to transfer electricity, which stunts the microbe’s ability to eat methane.
Previous research has overlooked this distinction due to how scientists measure iron levels in freshwater systems. The solid form of iron that becomes a part of rocks is known as ferric iron and can be directly measured by extracting the metal and visualizing the amount present. However, this method does not take into consideration the type of relationship it had with its rock and lends no information about the accessibility of the iron to microbes. Since these measurements are standard, this distinction has been widely missed in previous research.
Friese and colleagues ultimately unveiled the potential for a world-wide lack of methane consumption in iron-rich freshwater lakes by developing and applying a way to study iron in context with its relationship to rocks. Methane is an incredibly potent greenhouse gas and is widely produced in freshwater lakes, so lack of microbial consumption using iron allows for much of the gas to escape into our atmosphere, contributing greatly to our planet’s warming. Researchers currently don’t know the global extent of this surprising chemistry. This process may change how scientists understand methane production in natural systems and predict trends related to climate change.
Rust to the Rescue? by Jessica Buser-Young is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.