Featured image: A silver Roman Denarius, featuring the likeness of emperor Marcus Aurelius. CC BY-SA 3.0 via Wikimedia Commons
Authors: Jean Milot; Janne Blichert-Toft; Mariano Ayarzagüena Sanz; Chloé Malod-Dognin; Philippe Télouk; Francis Albarède
The Roman Empire was a superpower thousands of years ago, and with great power comes great (fiscal) responsibilities, including minting the money. To mint silver coins, the Romans needed vast amounts of silver, which historians and archeologists believe originated in the Iberian Peninsula, or present-day Spain and Portugal. However, the geologic origin of that silver is unknown as the depleted mines were abandoned long ago.
Silver is a rare metal– there is almost a thousand times as much copper as silver in the crust, and a million times as much iron. Because of this, most of the Earth’s silver is found in other minerals, especially sulfides. Galena, or lead sulfide (PbS), is one of these minerals, and has several important deposits in the Iberian Peninsula. Geochemist Jean Milot and colleagues recently studied silver isotopes from different galena regions in Iberia to try to locate which deposits might have contributed to the Roman coins archaeologists have found.
A rock sample containing the mineral galena, dark gray, and the mineral pyrite, golden. CC BY-SA 3.0 via Wikimedia Commons
Galena is a dark gray mineral with a distinctive cubic crystal form and is an important source of lead. However, Milot, at al. were more interested in galena that formed in a silver-rich environment, called argentiferous galena. Under these conditions, two lead cations can be replaced by the combination of a copper or silver cation and an arsenic, bismuth, or antimony cation.
Argentiferous galena deposits are helpful in trying to trace silver origins because silver isotopes are hard to tie to specific geological formations. For some elements, fractionation, or the separation of lighter and heavier isotopes, is driven by a single process, but for silver, fractionation occurs based on the source of silver-rich magmas, the amount of oxygen present, and other chemical factors. Because silver is a complex system, linking isotope ratios in silver to specific locations or deposits is nearly impossible, since predicting fractionation is difficult. The research team sidestepped this problem by using previous data on lead isotopes that contained eleven distinct galena deposits in the Iberian Peninsula. They studied the silver isotopes at these sites and compared that to the silver in the coins, letting them see which locations might have been sources.
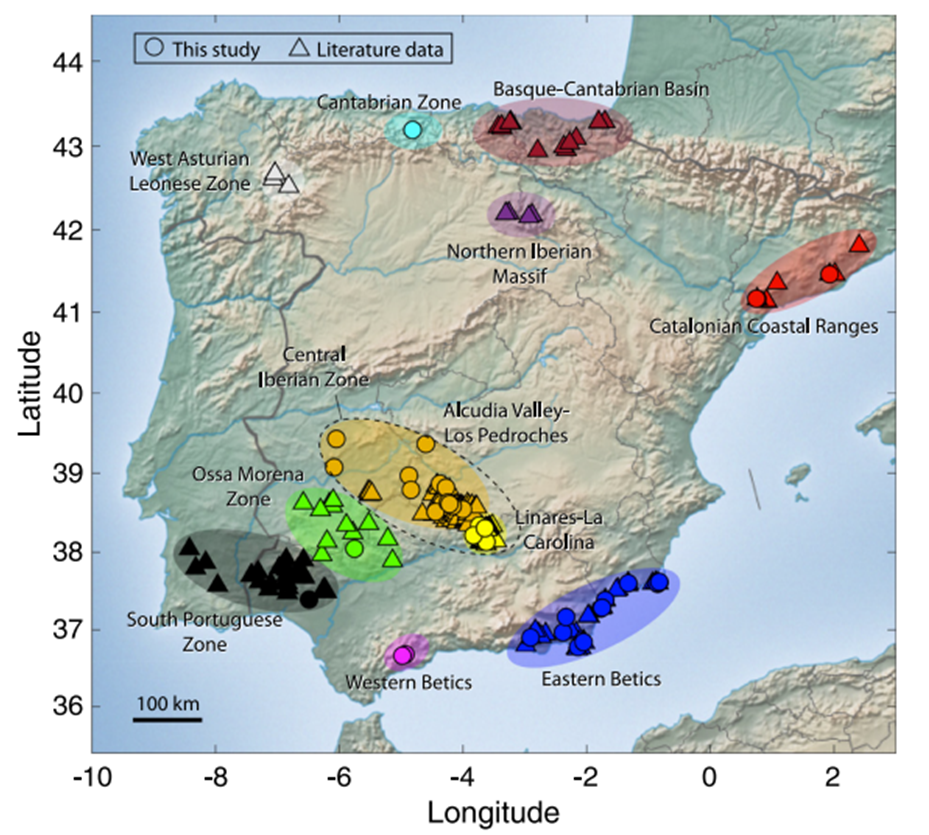
Map of regions with significant amounts of Galena in the Iberian Peninsula. The map displays locations with data from previous work (triangles) and data introduced in this paper (circles). Image from Milot, et. al.
Worldwide, the mixture of light and heavy silver isotopes in coins is pretty constant, showing only minor depletion or enrichment in 109Ag, the heavier isotope. However, the galena deposits the team studied showed a much wider range in isotopes, even within a site, diverging enormously from the range in coins. Some of the areas with the highest silver concentrations were only a little bit lighter than Roman coinage, but none were an exact match.
While the data are not conclusive, the team found the most similar lead isotopes were from samples in different parts of the Eastern Betics area, which makes it the leading contender as a source for Roman coinage. However, since silver isotopes fall into a tight band in coins around the world, not just coins minted from Iberian silver, they concluded that further work is needed to understand how smelting and other refining processes might homogenize or otherwise change isotope ratios.
Silver has been an important metal throughout history and has been widely used in coins since the seventh century BC, nearly three thousand years ago. The connection between Rome and the silver of its coin is immortalized in the chemical formula of the element: Ag, from the Latin argentum. While the authors were unable to determine the precise deposits mined, they used a creative approach to overcome some of the issues with studying silver isotopes, and uncovered clues that brought them one step closer to solving the mystery of the ancient Roman silver mines.
“Silver Doesn’t Grow on Trees: The Quest for the Ores that Formed Roman Coinage” by Sean Boyce is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.

