Featured Image: Eastern Scheldt Estuary near Zeeland, Netherlands. Photo courtesy Wikimedia Commons/ Luka Peternel, CC BY-SA 4.0 license.
Paper: Carbon and Hydrogen Isotope Signatures of Dissolved Methane in the Scheldt Estuary
Authors: Caroline Jacques, Thanos Gkritzalis, Jean-Louis Tison, Thomas Hartley, Carina van der Veen, Thomas Röckmann, Jack J. Middelburg, André Cattrijsse, Matthias Egger, Frank Dehairs & Célia J. Sapart
Estuaries are dynamic coastal environments where freshwater and saltwater collide and mix. Across the world, estuaries regularly have higher methane concentrations in the water than would be expected from equilibrium with the atmosphere. If the water was in equilibrium, or at a happy balance, with the atmosphere, then there would be no net transfer of methane to the atmosphere. Because there is more methane than expected in the water, estuaries are a source of this potent greenhouse gas, methane (CH4), to the atmosphere. The problem is that the processes leading to the excess methane in the estuary’s surface water are not well known in many European estuaries.
Thankfully scientists can use the isotopes of the carbon and hydrogen that make up methane molecules to tease apart the source and history of the methane present in the water. Imagine isotopes as different kinds of M&Ms. M&Ms all have similar amounts of chocolate, but the weights vary because of the fillings. In a bowl together, the plain M&Ms will more often be at the top and will be eaten first. Heavier varieties with fillings (peanut butter, peanuts, crisp, or hazelnut spread) will sink a little and be eaten more slowly, or at least it will take more effort to get to those M&Ms.
Similarly, isotopes of the carbon and hydrogen atoms that are in methane have differing weights. Methane molecules are made up of carbon weighing 12 (12C), 13 (13C), or extremely rarely 14 (14C) atomic mass units. Methane molecules can also have hydrogen weighing 1 (1H), 2 (2H also called deuterium), or very rarely 3 atomic mass units (3H also called tritium). Lighter isotopes 12C (comprising ~98.89% of Earth’s carbon a) and 1H (~99.97% of the Earth’s hydrogen b) are both produced and consumed more easily by microbial communities.
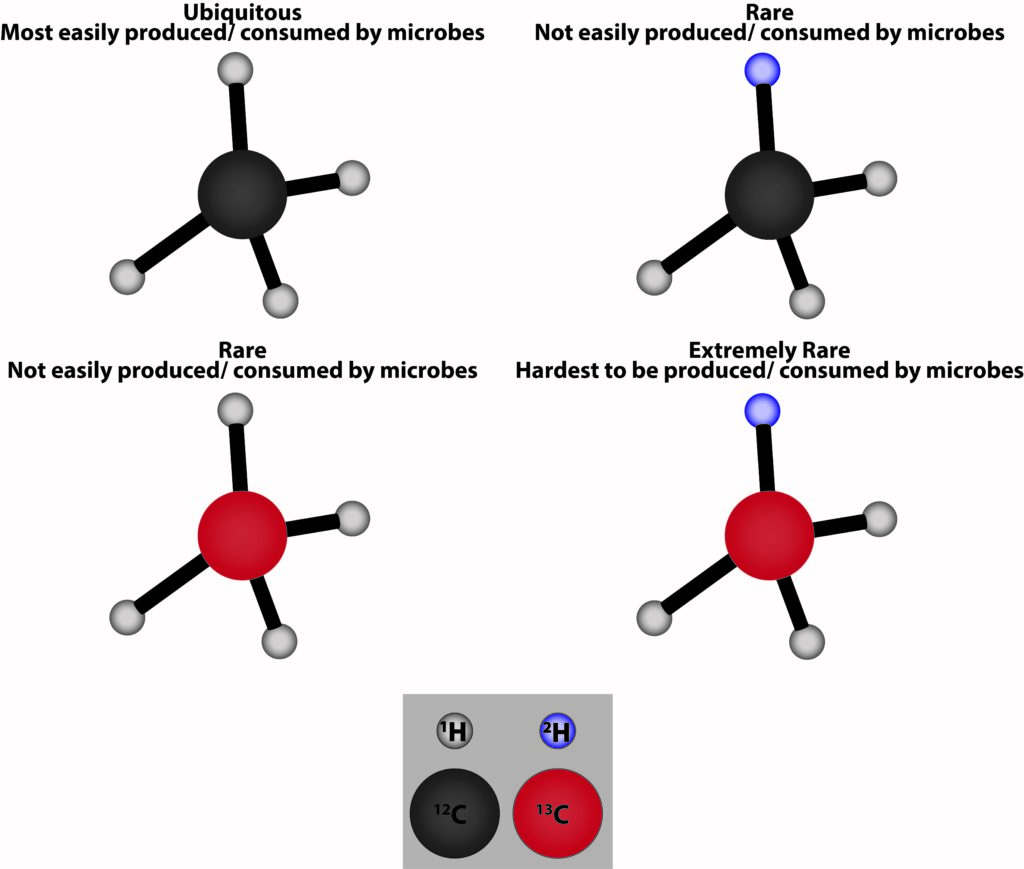
In a recent study, Jacques and co-authors measured the stable isotope ratios of 12C to 13C and 1H to 2H to unlock the mystery of where the elevated methane concentrations in surface water come from in the Scheldt Estuary. The Scheldt Estuary sits at the border between Belgium and the Netherlands on the North Sea.
The researchers found that methane in the lower estuary, from the Belgian-Dutch border to the North Sea, has isotope ratios concurrent with microbial production. With extensive tidal marshes and mud flats, methane is likely produced in the environments surrounding the lower estuary and then flushed into the system with the help of tides.
Whereas, the isotope ratios in the upper estuary, where the Scheldt and Rupel rivers converge to the Belgian-Dutch border, indicate that a majority of the methane has been removed by microbial consumption. While methane from natural gas seeps can have similar isotope ratios, seeps are absent in the study area. Therefore, Jacques and colleagues concluded that the microbial consumption of methane occurred either 1) upstream of the study sites, 2) in groundwater before it enters the upper estuary, or 3) by rapid and extensive (up to 80%) methane removal in the upper estuary, though that is less likely due to the shallow water column.
In the end, Jacques and co-authors state that stable isotope ratios provide a clearer picture of the complex methane cycle within the Scheldt Estuary, but there is still more to be learned. Accurately understanding the methane cycle in the estuaries is essential to precisely estimate global methane emissions to the atmosphere for climate predictions. Often research leaves more questions than it answers, and this study paves the way for further research endeavors.

Isotopes Begin to Unlock the Mystery of Methane Source in the Scheldt Estuary by Hadley McIntosh Marcek is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.



